单颗粒气溶胶质谱仪对碱金属和铵离子的灵敏度
Information for the paper
Title: Relative Sensitivity Factors for Alkali Metal and Ammonium Cations in Single-Particle Aerosol Time-of-Flight Mass Spectra
Author: Gross, Deborah S.
Year: 2000
Journal: Analytical Chemistry
URL: https://doi.org/10.1021/ac990434g
Introduction
- A wide variety of analytical techniques rely on the use of relative sensitivity factors (RSF's) for obtaining quantitative or semiquantitative results from multicomponent samples, including mass spectral and separation methods. These factors correct for differences in the response of the various species to the method being used, as well as for changes in the response of a particular species due to changes in the sample matrix.
- Equation 1 shows the definition of the RSF in a situation where the species present are detected as ions, where nix and nax refer to the number of ions and atoms, respectively, of species X present in the sample.
\[ RSF ( \frac{A}{B} ) = \frac{n_i^A/n_i^B}{n_a^A/n_a^B} \qquad (1) \]
- The observed ions can be used as markers of the chemical species present in the particles, indicating either the origin of the particles, the subsequent chemistry which the particle has undergone, or both.
- Not only do the peaks observed in the mass spectrum indicate the various chemical species present in the particle, but the relative intensities of the ions in the mass spectrum can provide quantitative information about the relative concentrations of these species in each particle.
- Factors such as the ionization potential of the species, the lattice energy of the specific salt analyzed, the absorption cross-section at the wavelength of the ionization laser used, and the interaction of the incident laser light with the particle can vary for each species and each particle.
- In this paper, we present an alternative method to address the variation in absolute peak area within a data set of many nonidentical particles.
- We demonstrate the applicability of this RSF to “real-world”particles by calculating the relative concentrations of Na+ and K+ in the mass spectra of individual ambient sea-salt particles
RESULTS AND DISCUSSION
Single-Particle Mass Spectra
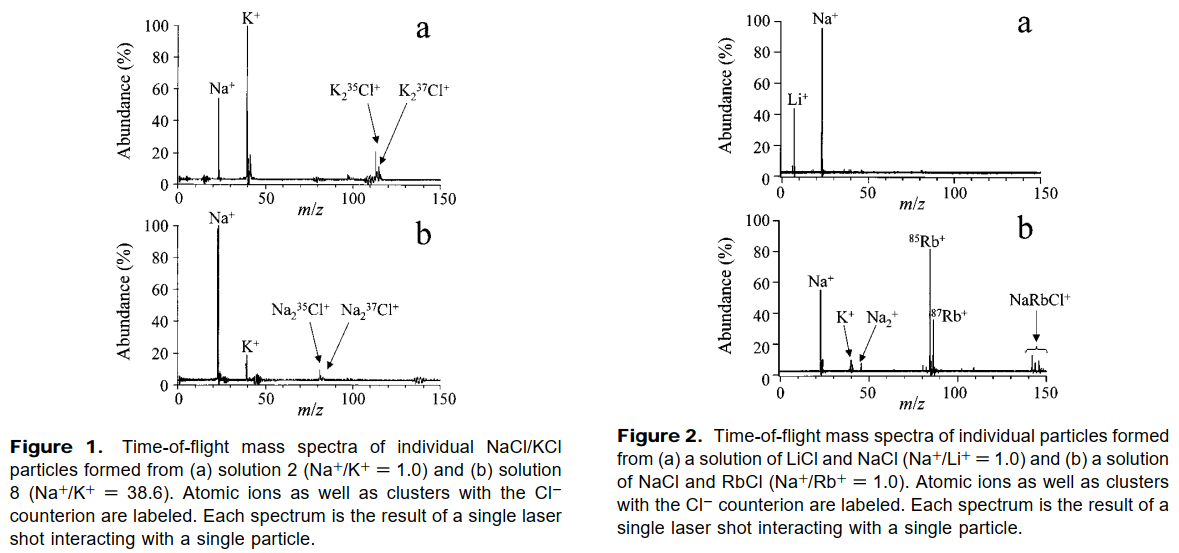
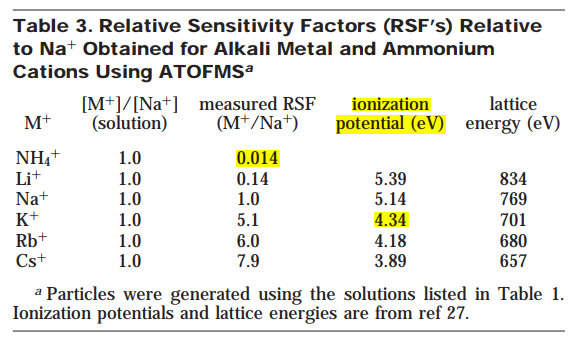
- It is evident from the mass spectra of particles formed from a solution containing known concentrations of different cations that the instrumental response to these cations is not identical, and thus RSF's are needed to better interpret the resulting spectra.
- Clearly the ratio of the relative intensity of the cation signals does not directly reflect the solution concentration ratios in any of these cases.
Reproducibility of Peak Areas.
- Typically, the measured absolute peak areas vary widely for particles sampled from a monodisperse aerosol, presumably because of inhomogeneities in the laser beam (i.e. differences in power density in the beam cross-section), which lead to differences in the energy imparted to the particles during the ionization process.
- However, the majority of the fluctuation in absolute peak areas depends only on the total area under all of the ion peaks in the mass spectrum, which increases if the ionization laser interacts with more of the particle and/or if a particle interacts with a higher energy spot in the laser beam. Currently, these two factors cannot be separated.
- Therefore, examination of the relative peak areas, in which the area of each peak is normalized to the total area under all peaks in the spectrum, should be more reproducible.
- Standard deviations of the average absolute peak areas range from 39 to 152% for Na+ and K+, over a sample of 200-300 “identical” particles, for the solutions listed in Table 2.
- The standard deviation of the average areas for each of these cations in most solutions drops to between 9% and 31%.
- These results demonstrate that the relative peak areas determined from single particle mass spectra are directly comparable, without the need for averaging multiple spectra. A comparison of the ratio of the relative area of any peaks within an individual spectrum to the ratio of the same peaks in another spectrum will provide relative quantitation to within ∼10-20% and will be applicable to individual ambient particles, provided that the effect of the matrix of the ambient particle is understood.
Determination of RSF's for Ion Pairs
Second, high-intensity peaks in the ATOFMS spectra typically exhibit some “ringing”, due to partially saturating the data acquisition system and/or signal reflections within the data acquisition circuitry, and this could mask low-intensity isotope peaks under certain circumstances. When this occurs, the peak area in a mass spectrum due to a particular ion is divided into multiple peaks. To calculate an accurate peak area in cases where this does occur, we use the sum of the relative area of all of the peaks +0.5 Da of the most abundant isotope. Thus, the calculated relative sensitivity factors should be independent of peak ringing.
The RSF for potassium versus sodium is determined from the inverse of the slope of the line for the singlet particles and is found to be 5.1 for this cation pair for the ∼0.8 µm particles.
- For the alkali metal cations, a clear trend is observed in that the ATOFMS shows a greater response to the heavier elements than the lighter ones.
- Both the lattice energy and ionization potential depend on the atomic/ionic radii of the ions considered; thus it is not surprising there is a linear relationship between the RSF and both parameters.
- It is obvious from the results shown in Table 3 that ATOFMS is much more sensitive to all of the alkali metal cations than to the ammonium ion.
- Our measured RSF for the Na+/NH4+ ion pair is 71 (a value of 0.014 for NH4+/Na+). This differs from the measured value published by Van Grieken who reports an RSF for Na+/NH4+ of 150. Differences in these values are likely due to a variety of factors, most obviously that the particles measured by Van Grieken contained three cations, NH4+, Na+, and K+, and therefore possess a different matrix. Van Grieken and co-workers determined the response of various cations in a mixed particle containing ions commonly found in sea-salt particles: NH4+, Na+, Mg+, Al+, K+, Ca+, and Fe+. The RSF's, with respect to NH4+, were found to be 1, 185, 10.3, 16.8, 660, 12.8, and 12.1, respectively. This implies a RSF for K+/Na+ of 3.6 in this matrix, compared to 4.2 in a three-component particle. Thus, the RSF's of Na+ and K+, relative to NH4+, change by 23% and 5%, respectively, as the particle matrix is changed. Other LMMS studies have shown dramatic and varied matrix effects, with quantitation possible only within a factor of 5, or within less than 50% relative standard deviation. Clearly, variation of ion signal response as a function of particle composition, or matrix, has the potential to affect the applicability of our measured RSF values.
总结与启发
- 质谱法、分离法等多种分析技术从多组分样品中获得定量或半定量数据时,都会使用相对灵敏度因子(relative sensitivity factors, RSF) 来校正各物质对所用方法的反应差异,以及由于样品基质的变化而导致的特定物质的反应变化。
- 各物质的电离势能、晶格能(盐类物质)、对所用电离激光对应波长处的吸收截面等不同;环境大气中每个颗粒物的物质组成可能不同,颗粒物与入射激光的相互作用也存在差异。这些因素都可能会对 RSF 产生影响。
- 在由相同溶液形成的单分散气溶胶粒子的单粒子质谱中,单颗粒气溶胶质谱仪(ATOFMS)测得的所含物质(Na+ 和 K+)的绝对峰面积变化幅度巨大。这种现象可能是由于激光束横截面中的功率密度不均,在电离过程中赋予颗粒物的能量不同,从而导致绝对峰面积变化巨大。在测定大量颗粒物时,激光打击到的颗粒物数量越多,即打击率越高,以及颗粒物与激光束发生相互作用的能量点的能量越高,则测得的平均绝对峰面积之和就越大。激光发射功率的变化同样会有影响。
- 绝对峰面积波动的情况,可以通过使用相对峰面积来将影响因素最小化。在由相同溶液形成的单分散气溶胶粒子的单粒子质谱中,给定离子峰的绝对峰面积平均变化59%,而相同质谱中的相对峰面积平均变化仅为16%。
- 对于碱金属阳离子,观察到明显的趋势是 ATOFMS 对较重元素的响应比对较轻元素的响应更大。
- 单颗粒气溶胶质谱仪(ATOFMS)对碱金属的灵敏度要远高于铵离子。
- 颗粒物组成不同,即基质不同,测得的 RSF 也不同。也就是说,颗粒物组成的差异会导致单颗粒气溶胶质谱仪(ATOFMS)对离子的灵敏度变化。
- 本文对单颗粒气溶胶质谱仪原理介绍的部分值得在写作中借鉴。
- 深入理解这篇文献需要一定的分析化学的知识,间接提醒了我,需要复习并深入理解质谱原理、激光原理、化学物种的电离势能、吸收截面等各种相关理化性质。
扩展阅读
- Wood-1998-TrAC: 质谱图解析参考
- Otten-1987-ACA: LAMMA解析