LAMS观测冬季污染过程中气溶胶的变化。
Information for the paper
Title: On-line Analysis of Urban Particulate Matter Focusing on Elevated Wintertime Aerosol Concentrations
Author: Tan, Phillip V.
Year: 2002
Journal: Environmental Science & Technology
URL: https://doi.org/10.1021/es011448i
Introduction
- These meteorological conditions in an urban center are associated with aerosol chemistry that is distinct from the summer season. The most pronounced changes in PM2.5 chemistry are a significant drop in particulate sulfates, and an increase in particle-associated nitrate during the winter.
- Wintertime nitrates have been associated with ammonium as ammonium nitrate. Particulate NH4NO3, which is volatile and possibly lost to evaporation during the summer, favors the particle phase at cold temperatures where nitrates increase from <10% to ∼20% of fine PM.
- Toronto is in close proximity to agricultural areas and their NH3 sources that possibly results in more NH4NO3 than other eastern North American cities.
- In contrast to nitrates, sulfates comprise >35% of PM2.5 in summertime while in winter they decrease to <25% of fine PM. This is believed to be a result of less oxidants in the winter, that would reduce hetero- and homogeneous SO2 oxidation.
- Other changes in general meteorological patterns during the winter such as a lower mixing height for the atmospheric boundary layer, changes in wind speeds / directions, and the presence of temperature inversions may also contribute to PM changes.
Experimental Section
- Due to issues such as partial ablation and matrix effects, the LAMS data were interpreted in terms of groups of particles to obtain a more representative description.
- When the results from the two polarities were combined, the negative polarity classes were scaled-up by a factor of 1.5, as indicated on those figures, to account for the higher sensitivity of cations (overall: 12 300 positive, 8400 negative particle spectra).
- The operational size range of the LAMS was found to be 0.3-3.0 µm, although only 0.52-2.5 µm particles were used for interpretation. The lower particle size limit applied was due to the low sensitivity of the instruments below 0.52 µm (see Supporting Information). While this may neglect a significant portion of the accumulation mode particles, considerable insights on particle behavior were still possible.
Results and Discussion
Temporal Trends
- The TEOM mass measurements were found to be lower than concurrent gravimetric PM2.5 due to volatile losses that have been quantified in the past to be approximately 23%. But to identify instances of relatively higher PM mass concentrations, the TEOM proved useful.
(A) PM Event A: December 15th, 10:30h
- Investigations of PM Event A revealed that the number concentrations of amine, nitrate, and cation organic particle classes increased up to the time when the APS and TEOM concentrations were at a maximum, while conversely that of the Ca and hydrocarbon classes decreased (Figure 3).
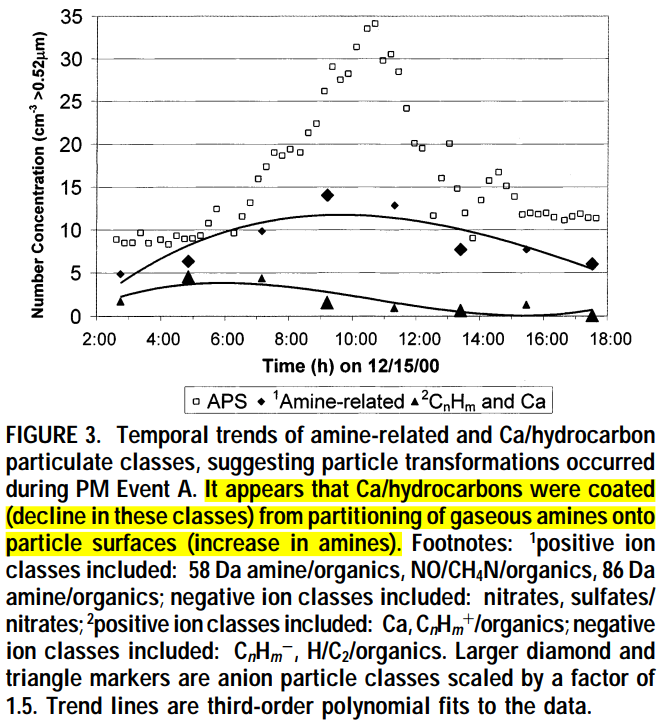
- The concurrent increase in amines, CH4N/NO, and other organic-containing particles detected in the positive ion mode, with nitrates and mixed nitrates / sulfates in the negative ion mode, suggests the formation of amine nitrates and sulfates on the particles.
- The simultaneous decrease in Ca, CnHm+/organics, H/C2/organics, and CnHm- suggests that these were the particle types that became transformed into amines.
- Inspection of the individual spectra in the CnHm+/organic and amine/organic classes during PM Event A revealed that the prevalence of ions in these mass spectra changed gradually over time. Tracking the CnHm+/organics class, at ∼3:00h, hydrocarbon type particles existed (Figure 4i) in low number concentrations (Figure 3). Afterward at ∼7:30h, some intermediate organic particle types were observed (Figure 4ii), characterized principally by the addition of m/z peaks of 58 and 86 Da. At this point, these particles were still classified as CnHm+/organics (Figure 3, CnHm and Ca peak). Finally by ∼10:30h, very few of the hydrocarbon type particles remained. Instead, particles with distinct amine spectra were evident (Figure 4iii). The presence of these intermediate particles suggested that the original hydrocarbon particles were transformed into amine-type particles.
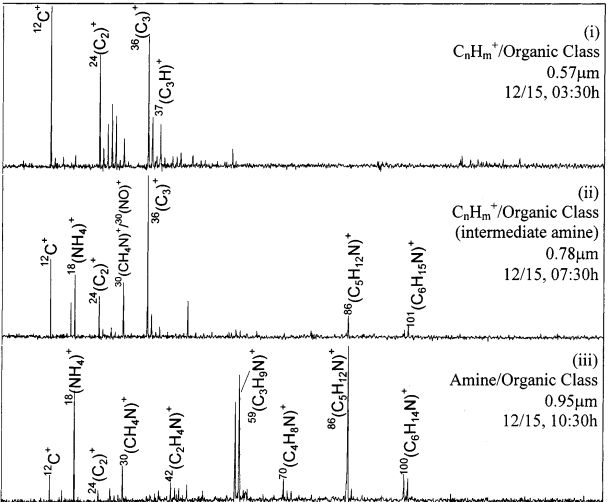
- The presence of ammonium, an ion with a low relative sensitivity factor, suggested that significant amounts of the ion were present on the particle surfaces, and gaseous NH3 was a likely precursor involved in PM Event A. The ammonium was most likely present as ammonium nitrate and sulfate (as supported in Table 2 by elevated sulfate- and nitrate-containing classes)
- The 58, 59, 86, 100, and 101 Da peaks were attributed to 58(C3H8N)+, 59(C3H9N)+, 86(C5H12N)+, 100(C6H14N)+, and 101(C6H15N)+, respectively, from a diethylethanoloamine salt, triethylamine or diethylamine based on similar spectra reported in the literature.
- It is possible that the particulate amines were formed through gas-to-particle partitioning of gaseous amines onto the surface of existing particles.
- Alternatively, although perhaps less likely, the amines may have been a secondary organic aerosol produced through a surface reaction of possibly: (1) NH3 with carbonaceous components on a particle, or (2) gaseous organic NOy with acidic particles.
- Amine classes were also evident on days with lower PM as indicated in Figure 1.
(B) PM Event B: December 20th, 02:00h
In the amine mass spectra from PM Event B, 58(C3H8N)+ and 59(C3H9N)+ ion peaks were intense in the amine group instead of the 86(C5H12N)+ peak that was a base peak in the amine class of PM Event A. Furthermore, there was a noticeable absence of the 100(C6H14N)+ and 101(C6H15N)+ amine markers from most of the amine particles in PM Event B. It is not clear if these more subtle differences indicated that the amine particles in PM Event B were truly different than those of PM Event A.
(C) PM Event C: December 20th, 23:00h
- The LAMS results suggested that this episode possibly originated from a wood combustion source.
- These hydrocarbons are associated with primary combustion, and their increase was concurrent with an increase in PM2.5 carbon concentrations of EC and OC.
- During PM Event C, the ratio of the number of particles in the K and K/organics classes to those in the Fe class (characterized by dominant Fe, and usually smaller K) showed a substantial K enrichment. An enrichment in the K to Fe mass concentration ratio, above its level for crustal materials, has been used in filter-collected PM analyses to indicate combustion. For LAMS, the ratio of K to Fe classes was 14 at 16:00h, December 20th, a time prior to the PM event. This ratio rose to 108, at the local maximum concentration which occurred at 00:00h on December 21st. K to Fe ratios after the peak, and during other times in this study, including PM Events A and B, were <26. Combining this K-enrichment information with the fact that K/hydrocarbons and sulfate were the main concentration changes during the episode (in contrast to the PM Event A which showed concurrent increases and decreases of particle types, suggesting gas-particle reactions) pointed to a primary particle source emitting K/hydrocarbons and sulfate.
Good words and Sentences
An aerosol laser ablation mass spectrometer (LAMS), a tapered-element oscillating microbalance (TEOM), and an aerodynamic particle sizer (APS) were operated in parallel to characterize the PM on-line.
Temporal analyses of this episode revealed chemical transformations as the amines, characterized by m/z peaks 58(C3H8N)+, 86(C5H12N)+, and nitrates, increased in number concentration while Ca and hydrocarbon particle classes concurrently decreased.
What this article inspired me
- 季节变化对颗粒物组分的影响。冬季硝酸盐组分占比高于夏季,硫酸盐组分占比反之。作者认为影响因素可能是冬季大气中氧化性组分较少。根据我以前阅读的文献,影响因素还包括夏季温度高,导致硝酸盐挥发分解等。
- 污染事件A中,有机胺颗粒物数浓度增加,同时,钙/烃类颗粒物数浓度减少。这是一个非常有趣的现象。作者认为可能是气态胺通过气/粒分配覆盖在了钙/烃类颗粒物表面。作者进一步分析了污染发生时段有机类颗粒物的质谱特征,发现随着污染加重,谱图中有机物组分逐渐减少,有机胺组分逐渐增多,说明原始含碳氢颗粒物向有机胺颗粒物转变。这个结论对我的启发有两点:一是为我发现的钙簇离子与有机胺离子的混合提供了依据;二是这个现象间接说明颗粒物中的有机胺除了一次排放污染源外,还有可能产生于污染过程中的有机化学反应,如气态有机胺的气粒分配等。作者通过在颗粒物中发现的铵根离子,说明氨气可能是这个反应的前体物。
- 污染事件A中,随着化学组分的转变,颗粒物粒径也在逐渐增大,从0.57到0.78到0.95 μm.
- 文中原句 "The presence of ammonium, an ion with a low relative sensitivity factor...", 这句话引自 Gross-2000-AC,单颗粒气溶胶质谱仪对铵根离子的响应较差。对颗粒物其他化学组分的响也有差异,如对钾离子的多度响应问题等。
- 污染事件C,含 K/Org 颗粒物数量与含 Fe 颗粒物数量的比值,间接证明了燃烧源的贡献。