云雾过程促进三甲胺的气-粒分配。
Information for the paper
Title: Cloud and Fog Processing Enhanced Gas-to-Particle Partitioning of Trimethylamine
Author: Rehbein, Peter J. G.
Year: 2011
Journal: Environmental Science & Technology
URL: https://doi.org/10.1021/es1042113
Introduction
Basic information of amines
- Amine concentrations are highest near regions of intense agriculture where they have been reported to be as high as 970 μg/m3 (∼250 ppbv) but are generally lower in urban areas with concentrations ranging from 0.16 to 2.8 nmol/m3 (∼10-70 pptv).
- Global emission of amines from animal husbandry operations have been estimated to be about 95-198 Gg nitrogen/year, with trimethylamine (TMA) being the most prevalent.
- Furthermore, the gas phase lifetime for TMA is estimated to be only a few hours in the troposphere on the basis of the reaction rates of TMA with the hydroxyl radical.
How amines arise in the particle phase
Although the presence ofparticle phase amines is clear, uncertainty remains as to how they arise in the particle phase.
Some laboratory studies have shown that gas phase amines can react with oxidizing agents to form stable, nonsalt, aerosol products. These studies however, used amine concentrations near 100 ppb, well above typical ambient levels.
Other studies suggest that gas-to-particle partitioning with the formation of aminium salts is also an important pathway leading to the presence of amines in the particle phase.
Ge et al. compiled thermodynamic data pertaining to the partitioning of various amines to the particle phase including Henry's Law coefficients, the equilibrium coefficients for reactions with nitric and hydrochloric acids, and for the solubility of their respective salts. On the basis of this compilation, TMA appears to be one of the least favored amines to partition to the particle phase. Thus, field observations of the partitioning of particle phase TMA will help to constrain the environmental behavior of amine compounds in general.
Results and discussion
The average mass spectrum
- The negative spectra also contain the sulphuric acid ion cluster peak m/z -195 [H(HSO4)2]- indicative of particle acidity and a hydroxymethanesulphonate peak at m/z -111 [HOCH2SO3]- (most significant in the Winter data), a product of organic oxidation in the aqueous phase and an indicator of particles that have undergone fog processing.
- Hydroxymethanesulphonate forms when both SO2 (g) and HCHO (g) are dissolved into the aqueous phase, the SO2 forms sulphite and bisulphite ions, and the bisulphite ions react with the HCHO. Therefore, the presence of this peak implies that the particle has or recently had high water content along with some degree of acidity.
- Furthermore, since the spectra contain peaks from other amine species, it is likely that other amines in the troposphere behave similarly to TMA and, thus, also appear in the same particles.
Influencing factors for concentrations
Increased concentrations of the TMA-containing particles were observed during periods of high relative humidity and fog events, if the air mass arrived from over agricultural/livestock areas located to the west and south of Toronto.
The summer fog events all occurred for air masses arriving from the west or south. Hence, no conclusion regarding the relative importance of fog versus the air mass origin can be drawn from the summer data.
In the winter data, the concentrations of TMA-containing particles spiked during fog periods, but they did not appear exclusively during fog.
In the winter, few TMA-containing particles were observed on clear days when the air arrived from the agricultural/livestock regions because there was no cloud processing to facilitate the partitioning of TMA to the particle phase, and few were observed on cloudy days when the air arrived from the north/east because there was no significant source of gas phase TMA.
The importance of cloud/fog processing is still evident, however, since the highest concentrations of TMA-containing particles were observed during periods of sustained cloudiness and when the air mass arrived from over the agricultural/livestock areas to the west and south, as shown in Table 1.

It should be noted that the ground is frozen during the winter rendering agricultural crop production an unlikely source of TMA. However, emissions from livestock remain throughout the year.
It is speculated that TMA containing particles are observed more frequently during winter due to greater cloudiness and lower mixing heights which result in lower altitude clouds and thus a greater opportunity for cloud processing as compared to the summer months. Cooler temperatures also appear to favor the partitioning of TMA to the particle phase.
One hypothesis to explain these observed trends is that TMA present in the gas phase partitions to the aqueous phase of preexisting particles during cloud/fog processing or during periods of high relative humidity.
Laboratory experiment
To test the partitioning of TMA to pre-existing particles and to explore the properties of the particles to which TMA will favorably partition, a laboratory experiment was devised where ambient particles passed through a high humidity reactor containing various concentrations of TMA as described in the Experimental Section.
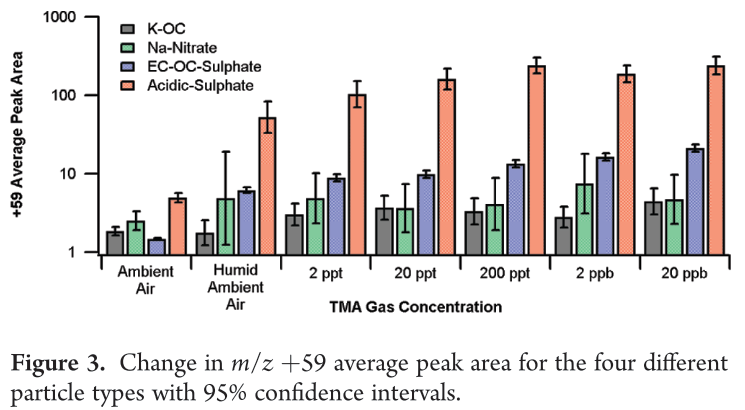
Denkenberger et al. has shown that the absolute peak areas of nitrate (m/z -62 [NO3-]) and sulphate (m/z -97 [HSO4]-) divided by the peak area for ammonium (m/z +18 [NH4+]) can provide a metric to compare particle acidity. This ratio was calculated to be ∼330 for the Acidic-Sulphate type compared to only 13 for the EC-OC-Sulphate type, further demonstrating the high acidity of the Acidic-Sulphate particle type.
The preferential partitioning of TMA to the acidic types was due to the formation of thermodynamically stable aminium sulphate salt while the plateau that begins at a TMA concentration of about 200 ppt suggests saturation as the particles became neutralized.
It should be noted that, for the EC-OC-Sulphate and Acidic-Sulphate particle types, the m/z +59 peak areas also increased when the relative humidity increased without adding any additional gas phase TMA to the system suggesting that adding an aqueous layer to the particle allowed more TMA already present in the urban ambient air to partition to the particle phase.
It has been hypothesized by Angelino et al. that high water content may assist in the dissociation of the aminium salts, shifting the gas-particle equilibrium to the particle phase.
Mechanism
Results from these ambient and laboratory measurements indicate that gas phase TMA partitions preferentially onto pre-existing particles in the 0.52-1.9 μm size range when the particle has a significant aqueous layer and when the particle is acidic. This mechanism can be described by the two-step process:
where the first equilibrium is based on Henry’s Law coefficient (KH = 9.6 mol/kg-atm at 25℃) and the second is based on the acid-base equilibrium coefficient (Ka = 1.6 × 10-10 at 25℃).
A total Henry’s Law coefficient has also been used to simplify theoretical calculations and is defined as the ratio of the total concentration of aqueous TMA (protonated and nonprotonated) to the partial pressure of gaseous TMA. At 25 °C, this coefficient increases from 6×103 to 6×107 mol/kg-atm as pH drops from 7 to 3, indicating that the partitioning should be favored in acidic particles.
TMA vs Ammonia
According to this mechanism, one would expect that ammonia, which is also basic and highly water soluble, should partition to the particle phase under similar conditions. Similar to the TMA-containing particles, concentrations of the ammoniumcontaining particles (defined as having a relative peak area greater than 0.04) were again highest when there was cloud cover and when the air mass arrived from over the agriculture/livestock regions.
The enhanced partitioning of TMA to the particle phase compared with ammonia during periods of cloud/fog processing can be explained in terms of thermochemical calculations.
TMA-nitrate vs ammonium-nitrate
Ge et al reported the gas-solid equilibrium coefficients for the evaporation of nitrate salts of ammonia and several amines including TMA. At -10°C, relevant for the winter season, this coefficient was approximately 2 orders of magnitude greater for trimethylaminium-nitrate than for ammonium-nitrate, and it was concluded that the formation of trimethylaminium-nitrate salts are unlikely in the presence of ammonia.
Contrarily, the partitioning of TMA to aqueous droplets or films should be possible even in the presence of excess ammonia, particularly at lower temperatures.
Specifically, approximate values for the total Henry's Law coefficients of 1.5×109 and 1.1×1010 mol/kg-atm were estimated for TMA and ammonia, respectively, at -10 °C and pH 3 based on data provided by Ge et. al. Thus, some aminium ions could be present in solution, even though most of the acidity might still be neutralized by ammonia.
It should be noted that, for 10-100 ppt TMA (g), the solution would likely not be saturated with respect to the trimethylaminium salt formation.
However, once the aqueous droplet begins to dry out, any trimethylaminium nitrate or chloride salts formed would evaporate back to the gas phase, although this could take some time during the cooler temperatures of the winter.
TMA-sulfate vs ammonium-sulfate
A second possibility is that a nonvolatile trimethylaminium sulphate would be formed. However, relevant thermochemical data for evaluation ofthis hypothesis are not available.
Conclusion
According to the aforementioned studies, for gas phase TMA to partition to the particle phase and be present in 0.52-1.9 μm particles, the following minimal conditions are required.
Gas phase TMA must be present, and higher concentrations of TMA lead to greater amounts in the particle phase.
Particles must have a significant aqueous phase.
More acidic particles will lead to higher concentrations of TMA in the particle phase.
Other
SO2 (g) emitted from the coal-fired power plants can produce acidic-sulphate aerosol, which then pass over the likely source of TMA.
On humid and cloudy days, the acidic particles will have high water content and some of the gas phase TMA will partition to the particle phase.
Since the main reported sink for TMA is the gas phase reaction with OH, the partitioning of TMA to the particle phase may extend the atmospheric lifetime and the distance that TMA can be transported.
总结与思考
好文章啊!主题明确,逻辑清晰,思维严谨。有外场观测,有实验室模拟,有机理解释。值得反复阅读的典范!
按照滤膜采样离线分析有机物(热-光分析)的方法,三甲胺(TMA)是有机碳(OC)的一部分,而 TMA 又易溶于水,因此,确切的说,单颗粒质谱仪鉴定出的 TMA-containing particles 应该是水溶性有机气溶胶的一部分。
TMA-containing particles 中,有机胺只是其中一种物质,从质量浓度来讲,甚至不是主要物质。主要物质是其他有机物和二次无机离子。所以,有机胺的来源和特性,对于 TMA-containing particles 的浓度是有贡献的,但是否是主要的贡献则有待与其他物质对比分析讨论。因此,云雾过程和农业/畜牧业区域对 TMA-containing particles 浓度的贡献,也有可能有其他水溶性物质的贡献,比如二次无机离子。补充 TMA 的峰面积与气团来源和云雾过程的响应关系应该更有说服力。
扩展阅读
- Whiteaker-2003-AE: Hydroxymethanesulphonate
- Denkenberger-2007-EST: calculate particle acidity by peak area.
- Ge-2010-AE: Thermodynamic properties and gas/particle partitioning